Cellular and Molecular Mechanisms of Respiratory Chemosensitivity
Stimulation of breathing by CO2
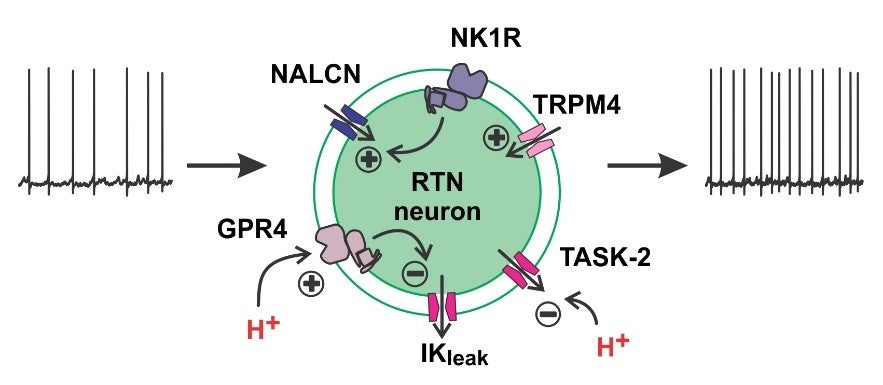
Stimulation of breathing by CO2 (or its proxy, H+) has been known since at least the turn of the 20th century. It serves as a homeostatic mechanism that matches CO2 elimination by lung ventilation with CO2 production by metabolism and rapidly regulates tissue acid-base balance. Although this regulatory system was described >100 years ago, the critical CO2/H+ sensors – the cells and their molecular H+ detectors – have been identified only recently. Our group has contributed to this by determining the phenotypic characteristics of specific brainstem neurons that are required for CO2-regulated breathing, and identifying distinct H+-sensitive receptors and channels that account for their intrinsic CO2/H+ sensitivity. Thus, a small group of pH-sensitive glutamatergic neurons in the retrotrapezoid nucleus (RTN) express the transcription factor, Phox2b, and the neuropeptide, Neuromedin B and are required for CO2-stimlated breathing. We subsequently discovered that GPR4, a H+-activated G protein-coupled receptor and TASK-2, a H+-inhibited K+ channel, are required both for pH sensitivity in RTN neurons and for CO2 regulation of breathing. In more recent work, we found a striking birth-related upregulation of expression for PACAP in mouse RTN neurons, and showed that this neuropeptide stimulates breathing and protects neonatal mice from breathing disturbances.
We believe these findings are clinically relevant. Mutations in Phox2b that render human patients insensitive to CO2 (leading to a condition called congenital central hypoventilation syndrome, or CCHS) also eliminate these RTN neurons when expressed in mice. In addition, genetic variants of PACAP have been associated in African-Americans with sudden infant death syndrome (SIDS), the leading cause of infant mortality in the early postnatal period.
Our ongoing work examines the receptor and ion channel mechanisms that regulate RTN neuronal activity and transmitter release, and single cell transcriptomic changes in those cells that occur during development and models of respiratory disease.
Select Publications
Review:
The retrotrapezoid nucleus: central chemoreceptor and regulator of breathing automaticity. Guyenet PG, Stornetta RL, Souza GMPR, Abbott SBG, Shi Y, Bayliss DA. Trends Neurosci. 2019 Nov;42(11):807-824. doi: 10.1016/j.tins.2019.09.002. Epub 2019 Oct 18. PMID: 31635852
Original Research:
A brainstem peptide system activated at birth protects postnatal breathing. Shi Y, Stornetta DS, Reklow RJ, Sahu A, Wabara Y, Nguyen A, Li K, Zhang Y, Perez-Reyes E, Ross RA, Lowell BB, Stornetta RL, Funk GD, Guyenet PG, Bayliss DA. Nature. 2020 Dec 2. doi: 10.1038/s41586-020-2991-4. Online ahead of print. PMID: 33268898
Neuromedin B Expression Defines the Mouse Retrotrapezoid Nucleus. Shi Y, Stornetta RL, Stornetta DS, Onengut-Gumuscu S, Farber EA, Turner SD, Guyenet PG, Bayliss DA. J Neurosci. 2017 Nov 29;37(48):11744-11757. doi: 10.1523/JNEUROSCI.2055-17.2017. Epub 2017 Oct 24. PMID: 29066557
Nalcn Is a “Leak” Sodium Channel That Regulates Excitability of Brainstem Chemosensory Neurons and Breathing. Shi Y, Abe C, Holloway BB, Shu S, Kumar NN, Weaver JL, Sen J, Perez-Reyes E, Stornetta RL, Guyenet PG, Bayliss DA. J Neurosci. 2016 Aug 3;36(31):8174-87. doi: 10.1523/JNEUROSCI.1096-16.2016. PMID: 27488637
Regulation of breathing by CO₂ requires the proton-activated receptor GPR4 in retrotrapezoid nucleus neurons. Kumar NN, Velic A, Soliz J, Shi Y, Li K, Wang S, Weaver JL, Sen J, Abbott SB, Lazarenko RM, Ludwig MG, Perez-Reyes E, Mohebbi N, Bettoni C, Gassmann M, Suply T, Seuwen K, Guyenet PG, Wagner CA, Bayliss DA. Science. 2015 Jun 12;348(6240):1255-60. doi: 10.1126/science.aaa0922. Epub 2015 Jun 11. PMID: 26068853
https://www.ncbi.nlm.nih.gov/sites/myncbi/douglas.bayliss.1/collections/48218324/public/
